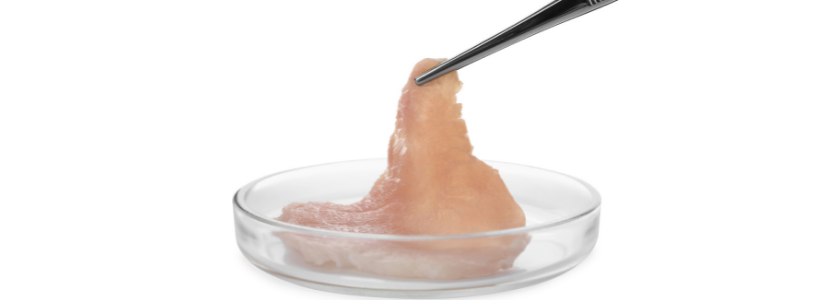
The U.S. Department of Agriculture’s (USDA) Food Safety and Inspection Service (FSIS) published today an advance notice of proposed rulemaking (ANPR) to solicit comments and information regarding the labeling of meat and poultry products made using cultured cells derived from animals under FSIS jurisdiction. FSIS will use these comments to inform future regulatory requirements for the labeling of such food products.
“This ANPR is an important step forward in ensuring the appropriate labeling of meat and poultry products made using animal cell culture technology,” said USDA Deputy Under Secretary for Food Safety Sandra Eskin. “We want to hear from stakeholders and will consider their comments as we work on a proposed regulation for labeling these products.”
On March 7, 2019, USDA and FDA announced a formal agreement to jointly oversee the production of human food products made using animal cell culture technology and derived from the cells of livestock and poultry to ensure that such products brought to market are safe unadulterated, and truthfully labeled. Under the agreement, FDA will oversee cell collection, growth, and differentiation of cells. FDA will transfer oversight at the cell harvest stage to FSIS. FSIS will then oversee the cell harvest, processing, packaging, and labeling of products. FDA and FSIS also agreed to develop joint principles for labeling products made using cell culture technology under their respective labeling jurisdictions. Seafood, other than Siluriformes fish, falls under FDA’s jurisdiction, whereas meat, including Siluriformes fish, and poultry, are
Keep up to date with our newsletters
Receive the magazine for free in digital version
REGISTRATION
ACCESS
YOUR ACCOUNT
LOGIN
Lost your password?