Content available at: Indonesia (Indonesian) Melayu (Malay) ไทย (Thai) Tiếng Việt (Vietnamese) Philipino
Infertility in breeder flocks has become a common concern in broiler breeder production.
- The causes of reproductive failure are multiple.
- Infertility could be related to females, but males have a more significant impact.
- Male fertility is a combination of proper spermatogenesis related to a healthy reproductive tract and mating behavior mainly linked to plasma testosterone levels. Both aspects have a high correlation with testicular size or weight.
- In Figure 1, we have a broiler breeder rooster’s normal healthy reproductive tract. The semen filling the ductus deferens indicates this rooster is in production.
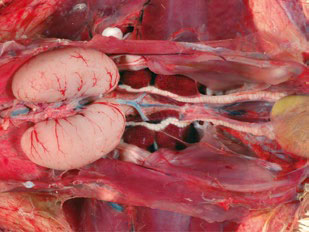
Excessive body weight gain as roosters age or poor conformation can also cause incomplete copulations in roosters and eventually a reduction in fertility. On the other hand, roosters with low body weight (< 3,800 grams) have been also associated with low fertility.
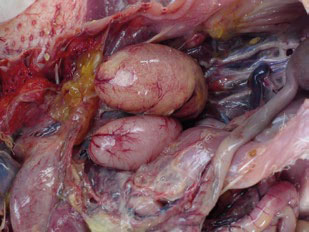
Male infertility increases as roosters age after 40 weeks but can be accelerated due to the following factors:
- Suboptimal development during rearing. Low body weights early in life cause weaker birds in the flock and lower placement in the pecking order. This causes stress, high blood corticosterone, reduced testosterone levels, delayed testicular development, and potentially faster testicular regression as roosters age.
- Extended exposure to constant photoperiods longer than 12 hours during rearing.
- Increase in photoperiod to more than 12 hours after 40 weeks of age.
- Marginal nutritional deficiencies during the rearing and mating phases.
- High crude protein and high calcium diets fed for a long time with levels similar to the ones observed in female diets can decrease sperm concentration in roosters older than 55 weeks.
- Diseases caused by infectious bronchitis virus (IBV), avian metapneumovirus (aMPV), avian influenza (AI), Mycoplasma gallisepticum and Mycoplasma synoviae (MG/MS), and bacteria like Escherichia coli (Figure 2), or Staphylococcus aureus(Figure 3).
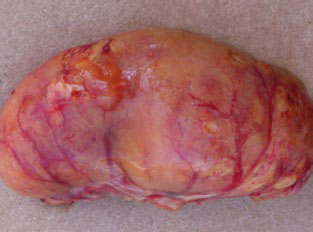
Figure 3. Orchitis caused by Staphylococcus aureus. Inflammation is evident in the swollen, discolored testis. Orchitis typically destroys the complete testis.
RESPIRATORY VIRUSES AND MALE INFERTILITY
Many respiratory viruses may also induce urogenital infections, leading to nephropathogenic disease, false layer syndrome in laying hens, epididymal lithiasis and epididymitis, leading to male infertility.
It has been reported that Arkansas (Ark) and Massachusetts (M41) IBV virulent strains can have venereal transmission (Gallardo et al., 2011).
- The IBV strain DMV/1639 has been detected in the efferent ducts of epididymides and testes in roosters in the USA (Gallardo et al., 2022).
- The QX-type IBV in Asia (Yan et al., 2023) and the European IBV genotype D274 replicate in Brazil (Villareal et al., 2007) have been isolated from the testis and ductus deferens, causing mass germ cell apoptosis and reducing fertility.
- IBV M41 and Ark were detected in spermatogonia and Sertoli cells of the testicles of almost all infected roosters seven days post-inoculation in the USA (Gallardo et al., 2011).

Roosters immunized prepubertally with some strains of the avian IBV have a high incidence of epididymal calcium stones, reduced daily sperm production and lower serum testosterone as adults (Jackson et al., 2006). It is essential to perform molecular surveillance of IBV to monitor vaccine strains and detect emerging IBV variants that can affect fertility.
EPIDIDYMAL LITHIASIS (STONES)
Epididymal lithiasis (stones) is probably the most common finding in males of broiler breeder flocks reporting increased infertility. Epididymal lithiasis is characterized by the formation of luminal stones rich in calcium in the rooster’s epididymal region (Figure 4).
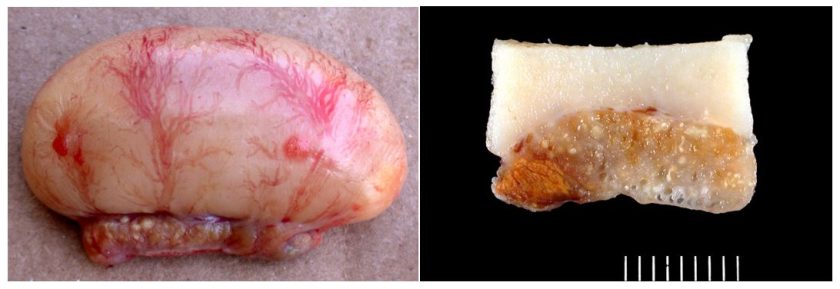
Males affected by this disease have severe testicular and epididymal alterations.
- Testicular damage encompasses the dilation of seminiferous tubules, sloughing of the seminiferous epithelium, and increased Leydig cell frequency within the interstitial tissue (Figure 5).
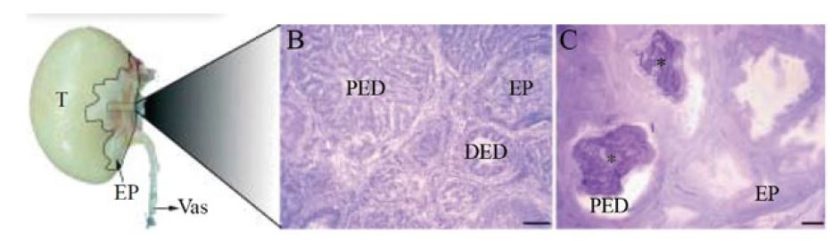
Figure 5. The epididymal region of roosters. (A) Macroscopical view of the testis and epididymal region (highlighted area). (B) The epididymal region of non-affected animals shows proximal efferent ductules with highly folded epithelium (PED), distal efferent ductules (DED), and epididymal duct (EP). (C) The epididymal region of roosters is affected by epididymal lithiasis, showing luminal stones (*) and loss of epithelial folding in proximal efferent ductules (PED). The epididymal duct (EP) shows no evident alterations. Bar in B and CZ100 mm. T, testis; EP, epididymal region; Vas, deferent duct. (Oliveira et al., 2011).
- The efferent ductules comprise up to 60% of the epididymal region and are the most affected segment in the rooster’s genital tract in this disease.
- These ductules are responsible for testicular fluid and calcium reabsorption, essential for sperm concentration and maturation.
- In roosters affected with epididymal lithiasis, there is an imbalance in Vitamin D (VDR) and estrogen receptors (ESR2) levels and epididymal tissue concentrations of vitamin D3, estradiol, and testosterone (Oliveira et al., 2011).
- These alterations interfere with paracellular calcium transport and calcium accumulation in the lumen of the ductules, which can cause calcium aggregation.
Additionally, scientists from the University of Kurdistan in Iran reported overexpression of the aromatase cytochrome P450 (CYP19) and aquaporin 9 (AQP9) in aged broiler breeder roosters.
- The AQP9 exhibited a 4.7-fold increase in expression, while the CYP19 showed a 1.17-fold increase in expression in roosters with genital stones compared to non-affected roosters (Heydari et al, 2023).
Aromatases, also called estrogen synthetases, are enzymes responsible for many reactions involved in steroidogenesis.
Roosters affected by epididymal lithiasis exhibited a notably high plasma estrogen/testosterone ratio, suggesting a correlation with the expression level of CYP19 (Heydari et al, 2023).
- Previous studies have shown a connection between elevated estrogen concentrations and rooster age, which strengthens the ability of the extratesticular ducts to absorb and concentrate estrogen.
- Estrogen plays a crucial role in regulating secretion of the sperm into the testis region and its subsequent reabsorption in the adjacent afferent ducts.
- This alteration in estrogen levels may promote increased fluid reabsorption in the epididymis.

The condensation of epididymal duct contents and the reduction in ciliated cells make it harder for semen to move. They can also block extratesticular ducts, which can lead to the low fertility syndrome seen in aged roosters (Figure 6).
- However, the reduction in fertility could also be attributed to changes in the production of sperm at the testicular level and impaired maturation in the epididymis.
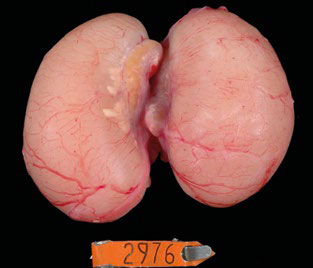
Figure 6. Testis of a 67-week-old rooster from a flock with normal fertility. However, the sperm has collected in the right testis and
epididymis because of an obstructed ductus deferens.
- Testes weight could be even higher in roosters with epididymal lithiasis than in non-affected roosters (Heydari et al, 2023).
- The seminiferous epithelium thickness and tubule diameter are reduced in affected roosters.
- Sperm motility and concentration decline, and sperm abnormalities increase in affected roosters (19.93 ± 2.17) compared to non-affected (11.93 ± 1.62) (Heydari et al, 2023).
Dietary antioxidants, vitamin C, E, selenium, and many phytobiotic products may mitigate some of the negative effects of aging, epididymal damage caused by viruses and bacteria. However, they do not prevent this condition and not always are effective.
A better understanding of this disease can help develop more prevention methods. Maintaining healthy testicles as roosters age may minimize fertility losses that have significant impacts on profitability.
References upon request
PDF










