Content available at: Indonesia (Indonesian) Melayu (Malay) ไทย (Thai) Tiếng Việt (Vietnamese) Philipino
Effects of Chronic Stress and Intestinal Inflammation on Commercial Poultry Health
- Modern broiler chickens are most certainly the most visible manifestation of genetic advancements.
- Genetic selection, food, health, and management measures carried out intensively have resulted in these successes.
- However, it is essential for production to keep the gastrointestinal tract (GIT) in good condition.
- As the growing period of broilers shortens and feed efficiency improves, the need for improved health and nutrition programs becomes more apparent.
- Because the changes during intestinal growth are minute, they are often overlooked, yet gut health impacts general health and productivity.
Currently, animal production systems require a constant search to reduce the effects of stress and chronic inflammation to improve energy use by producing animals.
Although there is no a “silver bullet” to prevent multifactorial conditions associated with chronic stress, several studies show improvement in intestinal microbial balance, metabolism, and intestinal integrity through alternative products such as:
- Probiotics
- Prebiotics
- Organic acids
- Plant extracts
- Essential oils
- Trace minerals
This being an international scientific trend, due to the anti-inflammatory, antioxidant and immunomodulatory effects, as well as improvement in intestinal integrity.
Substitution of antibiotics in production systems with alternative products, improved management practices, strict biosecurity, quality ingredients, absence of diseases (Mycoplasma/Salmonella), and effective immunization programs are efficient strategies for health objectives and productivity.
In this work, we focus on reviewing the significant repercussions of chronic stress and intestinal inflammation on the health and performance of commercial birds.
CHICKEN GASTROINTESTINAL TRACT ARCHITECTURE AND PLAYERS
In addition to absorbing and digesting water and food, the intestinal tract contains a diverse and complex microbial community (Celluzzi and Masotti, 2016), as well as an enteric nervous system (ENS) of metazoans considered the “second brain” of the organism (Schneider et al., 2019).
In addition to this complexity in the structure and microbial relationships, in chickens, the gut-associated lymphoid tissue (GALT) contains the highest concentration of immune cells in the organism, showing its relevance (Peralta et al., 2017; Casteleyn et al., 2010).
- Furthermore, the digestive system has primary lymphoid organs such as the bursa of Fabricius, where B lymphocytes originate and multiply; this component of GALT in avian species is essential for protecting the digestive system (Bar-Shira et al., 2003).
The intestinal microbiome can influence host biology, nutrition, immunity, and the neuroendocrine system (Dimitrov, 2011). GIT function appears to be mediated by short-chain fatty acids (SCFA) generated by bacterial fermentation (Wu et al., 2017), communication between the microbiota and neurons (Megur et al., 2020), the endocrine system (Fukui et al., 2018), the immune system (Maslowski and Mackay, 2011) and modulation of the intestinal epithelial barrier (Sharma et al., 2010).
- The ENS and endocrine gut network control the GIT motility and disruption in functional GIT disorders (Fukui et al., 2018).
Enteroendocrine cells (EECs) are found throughout the GIT epithelium and produce several hormones (Gribble and Reimann, 2019).
The first GIT hormones discovered were:
- Gastrin
- Secretin
- Cholecystokinin
- Insulin
- Glucagon
Over 50 hormones or bioactive peptides have been identified, making the GIT the primary organ displaying endocrine, neuroendocrine, autocrine, or paracrine activities (Rao and Wang, 2010; Gribble and Reimann, 2017).
In metazoans, intestinal enterochromaffin cells, a subpopulation of numerous EECs, produce 90% of the neurotransmitter serotonin (5-hydroxytryptamine) (Lund et al., 2018).
The intestinal microbiota partially controls the secretion of serotonin, dopamine, oxytocin, and endorphins produced by EECs (Forsythe et al., 2010; Liang et al., 2014; Mayer et al., 2014). There is great wisdom in the old saying “gut feelings”.
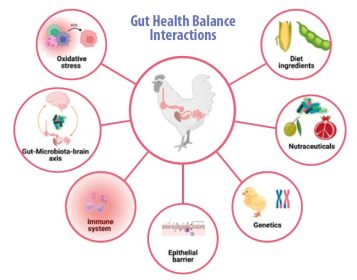
- Gut health balance or homeostasis is affected by the microbiota-brain-gut axis, the immune system, oxidative stress, nutrition, the intestinal epithelial barrier, genetic factors, and feed additives, such as nutraceuticals (Figure 1).
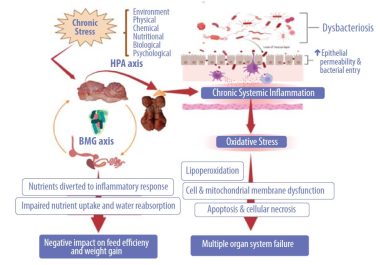
Prolonged stress and inflammation negatively impact the microbiota-braingut axis, causing dysbacteriosis and disrupting the tight junction proteins with systemic translocation of bacteria and other antigens (Figure 2).
During chronic stress and, as a result, chronic intestinal inflammation, energy for growth and reproduction is diverted away from these functions to sustain the inflammatory response. Poultry is no exception to this rule.
HPA AXIS
- The hypothalamic–pituitary–adrenal axis (HPA axis) is a complex network of direct influences and feedback interactions between three components: the hypothalamus, the pituitary gland, and the adrenal glands on top of the kidneys (Lu et al., 2021).
- The HPA axis is a significant neuroendocrine system that controls stress reactions.
- It regulates numerous physiological systems, including digestion, the immune system, mood and emotions, sexuality, and energy storage and expenditure in response to environmental stimuli (Cohen et al., 2006).
PATHOGENS AND DISEASES
- In general, constant damage or the presence of pathogenic agents induces a stress process in the GIT, increasing inflammation and, therefore, oxidative stress (Federico et al., 2007).
- Among the different possible pathogens in the GIT, we can include bacteria, and even protozoa, which infections can induce severe enteric damage, causing illness and high mortality rates.
- In chickens, Eimeria tenella is a primary cecal pathogen that negatively affects growth, feed utilization, and productivity due to necro-hemorrhagic typhlitis (Soutter et al., 2021).
- Another protozoon that targets the ceca is Histomonas meleagridis, the cause of histomonosis in chickens and turkeys, which is traditionally controlled with arsenicals in the diet.
- Alternatives to control protozoa infections include feed additives and other immunomodulators, adjuvants, and recombinant vaccine development.
- No commercial vaccines are available for H. meleagridis (Liebhart et al., 2017).
- There is an undeniable need to identify more effective solutions to lessen the impact of chicken coccidiosis and histomonosis.
- Yet, crucial resources still need to be included, making rapid progress difficult.
Bacterial infections are considered one of the main GIT-associated infections, which due to their infection process and presence, are inducers of acute or even chronic inflammatory processes (Yamamoto et al., 2013).
In murine models, Salmonella growth is aided, ironically, by acute inflammatory responses to pathogenic bacteria in the intestine, as there is increased migration of neutrophils and production of reactive oxygen species (ROS) and reactive nitrogen species (RNS) as a result of the Salmonella infection disrupting the balance of the microbiota (Winter et al., 2010a).
An increase in molecular oxygen in the lumen of the gut depletes important commensal anaerobes like Bacteroidetes and Clostridiales, which are essential butyric acid-producing bacteria (Rigottier-Gois, 2013).
The thiosulfate oxidation to tetrathionate is also a ROS by-product (Winter et al., 2010b). In murine models, it has been shown that Salmonella uses tetrathionate to strengthen its development (Winter et al., 2010b); tetrathionate broth is a component of enriched media for the culture of Salmonella in diagnostic laboratories.

However, a recent study has found that this differs for chickens (Saraiva et al., 2021).
- In contrast to murine models, in poultry, the ttrA and pduA genes do not appear to be significant virulence determinants in fecal excretion of invasiveness for Salmonella Enteritidis and Salmonella Typhimurium.
Interestingly, the deletion of both genes does not attenuate the pathogen but slightly decreases the numbers of Salmonella Enteritidis and Salmonella Typhimurium in caecum in poultry, reducing inflammation and allowing the bacteria to more easily invade gut epithelial cells and disseminate systemically, leading to severe clinical signs and higher mortality rates (Saraiva et al., 2021).
- Avian species are unique because they lack a urinary bladder, and renal discharge accesses the GIT directly.
- Both turkeys and chickens have a cloaca or common excretory pathway (Goldstein, 2006).
- Unlike mammals, where the kidney regulates the extracellular fluid composition independently, the urine flows into the cloaca in birds by a reverse peristaltic action to the ceca (Karasawa and Duke, 1995; Duke, 1999).
- Thus, the kidneys and lower GIT must regulate the extracellular fluid composition. Here, the avian ceca perform water reabsorption, fiber digestion (by bacterial fermentation), nitrogen recycling, microbial vitamin synthesis, and osmoregulation (Duke, 1982; Duke et al., 1983; Hall and Duke, 2000).
- The dietary composition can affect the moisture in fecal droppings.
- The water content of the feces directly impacts the litter moisture in poultry production systems, with moisture levels ranging from 15% to 44%.
- Poultry litter contains high levels of proteins and nitrogen (Kelleher et al., 2002). A significant issue with poultry litter is the loss of nitrogen, such as ammonia, during microbial fermentation of urea and uric acid (Nahm, 2003).
- In chicken houses, ammonia volatilization is one of the most stressful gases to poultry that severely affects the birds’ welfare, health, and performance (Moore et al., 2011; van der Hoeven- Hangoor et al., 2014).
DEFENSE NF-kB TNFA CYTOKINES CYTOKINE STORM
- Pathogen-associated molecular patterns (PAMPs) are elements of pathogenic bacteria that metazoans recognize.
- PAMPs include lipopeptides, peptidoglycans, and teichoic acids (Salminen and Isolauri, 2006).
- The endotoxin lipopolysaccharide (LPS), found in Gramnegative bacteria outer membranes, is a classic example (Kallapura et al., 2014).
- Generally, infectious agents (bacterial, protozoal, viral, helminth) stimulate host pro-inflammatory responses.
- For instance, in domestic poultry, coccidiosis may cause necrosis and inflammation in the intestine, resulting in fever, depression, reduced performance, and death depending on the Eimeria spp.
ROS AND RNS AND THEIR EFFECTS ON A MOLECULAR LEVEL
- Polymorphonuclear (PMN) leukocytes and macrophages fight pathogens as the first line of defense by producing reactive molecules capable of inducing oxidation or reduction reactions (Qureshi, 2003; Petrone-Garcia et al., 2021).
- These include ROS, such as superoxide, hydrogen peroxide, and hydroxyl radical.
- RNS comprise nitric oxide and peroxynitrite molecules.
- Both ROS and RNS are highly toxic to fight against invaders.
- They can penetrate the microbial cell wall easily, causing irreversible damage (Gostner et al., 2013).
- Immune signaling only initiates the production of these ROS and RNS molecules to intercept and kill pathogens (Sun et al., 2020).
- However, when ROS molecules overreact, they become immunotoxins capable of damaging the host cells and adjacent tissues, leading to severe local and systemic inflammation and multiple organ failure (Chen and Kevil, 2020).
- Due to this, its control is key to avoiding the adverse effect due to its overproduction and its negative impact (Lian et al., 2020).












