Animal Health & Pathology
Vaccinating for Marek’s? Don’t be Thrown Off by PFU Levels
To read more content about aviNews International March 2025
Animal Health & Pathology
To read more content about aviNews International March 2025
Content available at:
Indonesia (Indonesian)
With the use of vector vaccines growing across the globe, the topic of plaque-forming units (PFUs) continues to be a misunderstood term in the poultry industry.
Frequently, high PFU levels of herpesvirus of turkey (HVT) vaccines are inaccurately associated with greater protection; and worse, PFU levels are used to determine a vaccine’s possible dilution rate.
PFUs UNIQUE TO EACH VACCINE
The amount of PFUs per dose relates to how adapted the vaccine virus is to cell culture. Even though all commercial HVT vaccines are derived from the FC-126 strain isolated by Witter, each vaccine manufacturer has its own seed virus and will do several passages to get to commercial levels.
Depending on several factors (cell type use, days between passages, number of passages, and culture media, among others), viruses adapt differently to grow in cell culture.
The more adapted the virus is, the higher PFUs per dose can be obtained. On the other hand, better growth in cell culture might lead to less replication in vivo.
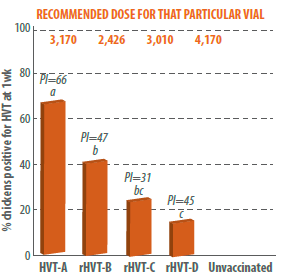
In this study, different HVT products including one conventional and three recombinant HVTs were administered in ovo at 1,500 PFUs regardless of what the recommended dose was for those vaccines.
The results demonstrated that by reducing the dose to 1,500 PFU, some vaccines performed better than others (Figure 1).