Content available at: Indonesia (Indonesian) Melayu (Malay) ไทย (Thai) Tiếng Việt (Vietnamese) Philipino
The Avian Influenza disease Virus (AIV) belongs to the species influenza virus type A, family Orthomyxoviridae, genus Alphainfluenzavirus based on the International Committee of Viral Taxonomy.
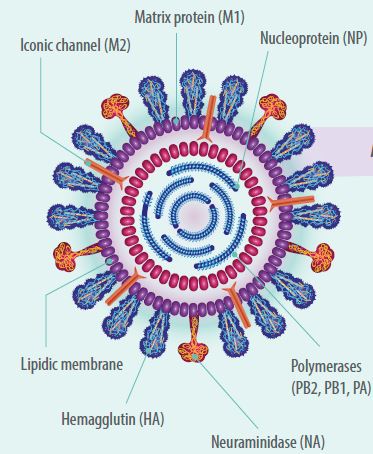
- The AIV is characterized by being surrounded by a phospholipid membrane and having a spherical or filamentous shape with a size of approximately 80-120nm.
- It contains a genome composed of 8 segments of linear single-stranded RNA with a 3-5’ sense (negative).
- The RNA genome codes for 11 proteins, nine of which are structural (PB2, PB1, PB1-F2, PA, HA, NA, M1 and M2) and two non-structural (NS1 and NS2).
The segments are:- Segment one codes for the polymerase enzyme PB2;
- Segment two codes for the polymerase enzyme PB1 or PB1-F2;
- Segment three codes for the enzyme acid polymerase PA.
- The fourth segment codes for the adhesion glycoprotein called hemoagglutinin (HA), which is involved in the binding of the virus to the cell, determines the degree of virulence, and is the antigen that allows the classification of influenza A viruses into 18 different HAs (16 HAs in birds and 2HAs in bats).
- The fifth segment codes for the nucleoprotein (N) and is the antigen that allows classification of influenza viruses by genus into A, B, C and D.
- Segment six codes for neuroaminidase (NA), which is a glycoprotein present on the surface of the virus that is involved in the release of viral particles from host cell receptors and is the antigen that allows the classification of influenza virus type A into 11 distinct NA (9 NA in birds and 2 NA in bats).
- Segment seven codes for the matrix (M1 and M2).
- Finally, segment eight codes for the nonstructural protein NS1 and NS2.
- The AIVs found in the bird class can express on their surface one of the 16 HA and one of the 9 NA that can theoretically give rise to 144 viral subtypes. These two proteins have antigenic variations through two mechanisms:
- The first is antigenic drift consisting of base mutations (substitution, insertion, deletion or reversal) due to the lack of correction of the RNA polymerase enzyme during viral genome synthesis.
- The second is by recombination of its segmented genes when a cell is infected by two different subtypes. The latter mechanism allows the virus to acquire genomic segments from other species such as pig and human.
NATURAL HOST OF AIV
Currently, the 16 HA and 9 NA are found in nature in coexistence with about 225 species of wild waterfowl distributed worldwide and belonging mainly to the order Anseriforme (ducks, geese and swans) and order Charadriiforme (gulls, terns and shorebirds).
- These viruses are also found in wetlands which are the aquatic habitat and where water is considered a way of transmission, but the few isolates in water suggest that it is limited.
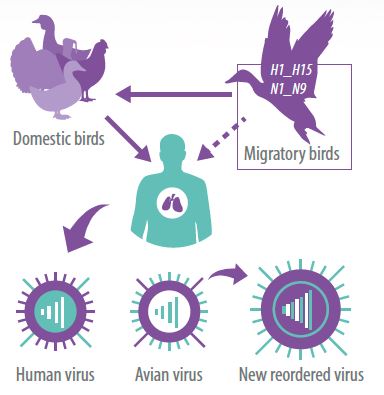
In North America, Germany and Switzerland, the subtypes with the highest frequency in ducks are H4N6 and H6N2, while H13-H16 subtypes have been reported in shorebirds.
However, some subtypes related to hemoagglutinin H5, H6, H7 and H9 that come from wild waterfowl as low pathogenic viruses and have constant contact with domestic poultry production systems such as ducks, chickens and turkeys; they begin a process of adaptation characterized by an increase in virulence and capacity to cause fatal disease.
An example of this is the emergence and and current presence of the highly pathogenic H5N1 subtype in poultry in China, which has shown its capacity to kill domestic birds, some wild birds, mammals and, on rare occasions, humans when in close contact with poultry.
- Where the virus has been detected in cases of pneumonia in humans in Asia, the genome has an avian origin, but the virus has not evolved to transmit directly from human to human.
Another example of zoonosis has been the H7N9 subtype that was detected in 14 fatal cases of 63 humans in China.
- The genome of this subtype is genetically related to poultry viruses in live bird and pigeon markets.
- Although the H5N1 and H7N9 subtypes have been phylogenetically associated with poultry, close contact is a key factor, as well as other humanspecific health factors.
The World Health Organization also considers H9N2 to a lesser extent, which is of low pathogenicity in chickens, but has been detected in humans.
PATHOGENESIS OF AVIAN INFLUENZA VIRUS H5N2 OF MEXICAN LINEAGE
The pathogenesis of the first Mexican AIVs of low and high pathogenicity were performed in vivo experiments. In these studies, it has been
evidenced that:
- AIV H5N2BP/1994 inoculated into 4-week-old domestic chickens causes low mortality with moderate respiratory signs.
- The virus spreads to the lung, lymphoid organs and visceral organs, causing tissue necrosis and lymphocyte depletion in lymphoid organs;
- AIV H5N2AP also spreads to other organs such as the heart, brain, trachea and cecal tonsils causing necrosis, hemorrhages and loss of cilia in tracheal epithelium.
- The cause of death of chickens experimentally infected with AIV H5N2AP is associated with damage to the capillary endothelium and thrombocytopenia leading to widespread vascular damage.
- Experimentally, it was also shown that the H5N2AP virus is present in the peripheral blood 28 hours after inoculation and up to 72 hours after the end of the experiment.
Pathogeny study
The study of pathogenesis of AIVH5N2 in other avian species such as duck, plover, turkey, pheasant and quail inoculated with the virus by the intravenous route do not become ill and do not infect to chickens free of specific pathogens that coexisted with the infected wild birds.
- In another study, the pathogenesis of AIV H5N2BP/2007 in domestic ducks experimentally infected by intranasal and intratracheal routes, showed that this virus does not cause clinical signs in the duck but can be isolated from turbinates, larynx, trachea and lungs in the first 48 hours after inoculation and cause inflammation with mild to moderate lymphocytic infiltration.
Excretion kinetics studies
Excretion kinetics studies of Mexican lineage AIV H5N2BP/2007 were detected by virus excretion in the oropharynx and cloaca at least 21 days after inoculation in chickens and ducks.
- While other authors have reported that inoculation of the H5N2BP subtype of Mexican lineage in ducks is excreted between two to fourteen days after inoculation by the respiratory route and from zero to seven days after inoculation by the cloaca.
It was also observed that AIV H5N2BP/2007 with mexican lineage inoculated in domestic duck can be excreted initially digestive and subsequently respiratory and vice versa in chicken.
Nucleotide mutations
Most of the nucleotide mutations studied in AIV have been concentrated in the HA gene cleavage region because the difference between the amino acid sequence of the HA of an outbreak virus and the vaccine virus can be determined, which decreases protection.
This characteristic shift in tropism between respiratory and digestive tissue has been observed in chickens and ducks inoculated with H7N3BP, H7N2BP, H7N3BP and H7N9BP from wild ducks, chickens and domestic turkeys. The explanation for the change in tropism can be associated with:
- Nucleotide mutations occurring in the HA gene and in the NA gene during viral replication and transmission in the animal as has been observed in the H11N9 subtype of wild duck.
- Or because it has genes from other virus subtypes with which there has been recombination between chicken and duck viruses; as is the case of subtype H9N2BP in domestic duck from South Korea and subtype H5N2BP in wild geese in Africa.

Accumulation of basic amino acids
The accumulation of basic amino acids in the HA cleavage region that is associated with high virus virulence in chickens is also analyzed, but the presence or lack of this basic amino acid region in some cases corresponds to viruses of low pathogenicity, suggesting the involvement of other genes in virulence, such as PB1-F2, PB2 and NS1 where mutations have been found by puric or pyrimidic base associated with virulence.
SITUATION OF AVIAN INFLUENZA VIRUSES (AIV) IN MEXICO
AIV H5N2
On May 23, 1994, the first isolation of low pathogenicity AIV H5N2 was reported, and in December of that year the first isolation of AIV H5N2AP in commercial poultry by the Laboratory of the U.S.-Mexico Commission for the Prevention of Foot-and-Mouth Disease and Other Animal Diseases (CPA). The AIV H5N2AP was eradicated in June 1995.
However, to date, AIV H5N2BP is prevalent in commercial farms and backyard hens, but because of its low pathogenicity it can go clinically undetected as birds can recover.
In 24 years of presence of the H5N2BP subtype, there are no reports showing that the virus has mutated to the highly pathogenic presentation, and its genome is of avian origin.
AIV H7N3
In June 2012, AIV subtype H7N3 with highly pathogenic molecular and biological characteristics appeared for the first time in poultry production units producing eggs for consumption in the State of Jalisco. After being controlled, it was epidemiologically absent for 17 weeks, appearing in January 2013, and present to date in some regions where there is technified poultry or backyard poultry farming.
- The complete genome of AIV subtype H7N3 has phylogenetic origin with AIV from wild ducks of the United States and not with humans or pigs.
The highly pathogenic AIV subtype H7N3 present in hens from the State of Jalisco was identified antigenically and molecularly in the conjunctivae of two poultry workers from this region who presented conjunctivitis without fever or respiratory disease.
- Experimentally, this unadapted viral isolate causes fatal disease in mice and is easily transmitted directly between ferrets, as well as easily replicating in human bronchial cells.
At present, subtypes H5 and H7 are avian influenza viruses that must be reported to SENASICA and OMSA. With respect to AIVs isolated or detected in wild birds in Mexico, low pathogenic type A influenza viruses H7N3, H6N2, H4N2 and H5NX subtypes have been reported in migratory wild ducks.

Currently, the H5N1 and H7N9 avian influenza viruses of asian lineage are the subtypes with the greatest impact on people’s health and their origin is due to the close contact between different species of production birds, wild ducks and the people who raise and trade them.