Content available at: Indonesia (Indonesian) Tiếng Việt (Vietnamese)
A few years ago, the key was found to produce HYPOCHLOROUS ACID IN SITU. One of the biocides that in recent years is being used more and more, because of its high oxidizing power, combined with its low application cost and its simple production in situ.
Water is an essential compound for life, to the point that life would not be possible without it, and it is also essential that it be chemically drinkable and microbiologically drinkable.
- Commission Implementing Regulation (EU) 2021/347 of 25 February 2021 approves active chlorine released from hypochlorous acid as an active substance for use in biocidal products of types 2, 3, 4 and 5.
- And Commission Implementing Regulation (EU) 2021/365 of 26 February 2021 approves active chlorine released from hypochlorous acid as an active substance for use in Type 1 biocidal products.
- In July 2022 the European Commission approved hypochlorous acid as an active substance for use as a Type 5 biocide.
- Biocides group TP5 Drinking water includes those biocides used for the disinfection of drinking water, both for humans and animals.
Advantages of using Hypochlorous Acid
- In addition to its advantages as a result of its broad bactericidal, virucidal, fungicidal, sporicidal and biofilm eliminating action in water pipes, it does not present the disadvantages that can occur when using other biocides such as hypochlorites and chlorine dioxide, as no harmful chlorinated residues are formed.
- It is effective and harmless to the environment, 100% biodegradable and safe to handle. Therefore, it is also perfect for use on ecological farms.
- The advantage of hypochlorous acid is its effectiveness, potency and capacity to be used as a natural disinfectant in different areas, being considered ideal because it has properties that make it highly effective in the area or surface to be treated (Severino, 2023).

Methods for obtaining Hypochlorous Acid
Hypochlorous Acid can be obtained through three different methods:
1. Chlorine gas hydrolysis
- Chlorine gas hydrolysis consists of the application of chlorine gas directly into water.
- This method is widely used in water disinfection processes for swimming pools, aqueducts and industries.
- However, the uses are limited, both because of the high concentrations of chlorinated species in solution and also because of the instability of the final product (Monarca et al., 2004; Lowe et al., 2013).
2. Hypochlorite acidification
- Because hypochlorite is commercially available, this method is widely used.
- It allows the highest generation of HOCl in solution, with a high redox potential, but undesirable toxic residues can be obtained.
- Unfortunately, in many cases the solutions obtained lack the stability necessary for prolonged use (Wang et al., 2007).
3. Salt solution electrolysis
- Commercially, the new salt and water electrolysis method is increasingly used.
- It allows the formation of stabilized hypochlorous acid in situ for its use, generating formulations with HOCl ideal for water disinfection processes, surfaces or sanitary medical devices.
- The method consists of using an electrochemical cell, composed of cathodes and anodes that transmit an electrical pulse to a homogeneous mixture of water and salt buffers.
- The electrical charge allows the increase of the oxidative potential of water (ORP >1000 mV).
- The electrical phenomenon also allows the dismutation of salts and the subsequent release in very small quantities of chlorinated species in solution, including: NaOCl and NaCl, although hypochlorous acid is considered the active ingredient of formulations obtained through this system ( up to 500 ppm ) (Innoue et al., 1997; Landa-Solis et al., 2005).
- Hypochlorous acid oxidants (HCIO) and hypochlorite (OCI) are formed at the anode.
- The pH of the solution is mostly neutral and the free chlorine solution (1 ppm) is dominated by hypochlorous acid, which is the one with the microbicidal power acting with an immediate and a permanent effect as the redox potential produced remains.
Hypochlorous Acid, a new era in drinking water disinfection
Hypochlorous Acid is also produced naturally by macrophages and neutrophils to fight infections, in what is known as a “respiratory burst” during the fight against pathogens (Weiss, 1989).
- This reinforces the fact that hypochlorous acid is one of the only non-toxic disinfection agents.
Its microbicidal spectrum is broad and effective, eliminating them quickly depending on the oxidation-reduction potential (ORP) value (ideally at least 650 mV).
To realize its activity, it should be noted that hypochlorous acid is 90 times more efficient at eliminating microbial pathogens than sodium hypochlorite (bleach) and 10 times more efficient than chlorine dioxide. The doses of use are also totally harmless to humans, animals and the environment.
- Redox Potential (ORP) is an effective measure of chemical oxidation-reduction energy by means of an electrode, converting it into electrical energy, which is used to determine the sanitation of drinking water.
- It is expressed in millivolts – mV – and tells us about the oxidation or reduction potential. It is actually a measure of the activity of the electron compared to the activity of the reference electrode, which always keeps the potential constant.
- The word “potential” refers to the capacity at the site of action. Potential energy is the energy stored and ready to be put into action.
- In addition, temperature compensation is not necessary in the measurement of the ORP and is independent of the ppm of the biocide.
In practical terms with current knowledge, the value of the oxidation-reduction potential for chlorinated biocides can be interpreted for action on bacteria from:
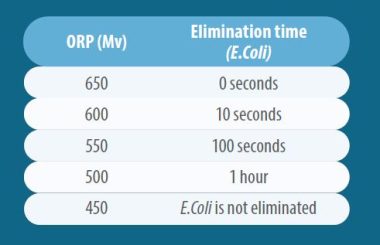
At 650 mV, viral inactivation is also instantaneous.
- In the case of treatment with hydrogen peroxide, the values will range between 250-275 mV when consumed with the reducing elements.
- The ORP measurement must be correct and must be made at any point of the installation.
GRAM NEGATIVE BACTERIA
- Gram-negative bacteria contain sulfur and heme (iron-rich) groups in their outer membrane that are essential for normal electron transport.
- An irreversible enzymatic reaction of HOCl with membrane proteins produces structural damage that alters cell permeability and affects bacterial viability (Rosen & Klebanoff, 1982; Mckenna & Davies, 1988).
GRAM POSITIVE BACTERIA
- In Gram-positive bacteria, hypochlorous acid differs in point of action, acting on the amino groups of glycine present in peptidoglycan.
- HOCl oxidizes and/or chlorinates endotoxins and exotoxins, neutralizing their action and also oxidizes cysteine residues in gingipains such as Rgp and Kgp.
- HOCl interferes with the C5 component of the complement cascade, which upon activation produces two fractions, including C5b with lytic activity on the bacterial cell membrane.
VIRUS
- In viruses, it acts by lipid peroxidation of the membrane covering the pathogen. The action occurs at only 500 ppm of free chlorine injection into the drinking water, unlike other chemical species such as chlorine dioxide and sodium hypochlorite which exceed 30,000 ppm of chlorine injection.
- Knowing that they all range between 0.5 and 3 ppm once in the animal’s consumption line, we can have an idea of the toxic chlorates, haloacetic acids and trihalomethanes that are produced in the respective reactions with water with the latter products.
Hypochlorous Acid has anti-inflammatory and tissue proliferation effects. It inhibits histamine, interleukin 2 and leukotriene 4.
- At low doses HOCl can activate metalloproteinase proforms (MMPs), collagenases and gelatinases. At high concentrations HOCl inhibits MMP-7, collagenase and gelatinase activity (Fu et al., 2003).
- It may be applied when authorized (soon), in wounds caused by feather plucking and pecking both on the skin and caruncles, to facilitate the disinfection of wounds and their healing.
The advantages of HYPOCHLOROUS ACID can be specified in:
- Very effective against gram + and gram -, viruses, fungi and highly sporicidal.
- Fast acting, 99.99% destroyed in seconds.
- Effective at low concentrations.
- Effective in presence of organic matter.
- Non-toxic and non-irritating.
- It is not corrosive to plastic or metallic materials.
- Total elimination of biofilm from pipes and drinking troughs.
- Permanent eradication of algae in drinking troughs.
- It is 100% biodegradable.
- Ecological biocide: provides natural water without toxic residues.
- It does not alter the smell or taste.
- Does not stain. Does not discolor surfaces or fabrics.
- Microorganisms do not develop resistance.
- Decreases mortality.
- Reduces medications.
- Reduces enteric processes.
- Reduces conversion rates.
- Inexpensive to produce and assured availability upon production.
The product does not have any negative effect on health and environment. It is classified as NON HAZARDOUS according to the European Union Regulation and authorized with biocide for the sanitization of drinking water (TP5).

In summary
The use of hypochlorous acid (HClO) to disinfect drinking water has several significant advantages:
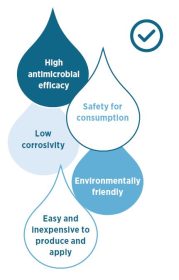
Problematic
There are currently several biocides used for the disinfection of drinking water that DO NOT COMPLY with the STANDARD of maintaining a stable ORP (Oxidation Reduction Potential) throughout the water supply line. In spite of partially achieving it, they do not reach the minimum value of 650 mV in all of it, leaving space for the microbial presence by not eliminating it totally or doing it slowly.
- This is why modern poultry farming, in spite of having excellent management standards, sometimes finds itself with outbreaks of diseases caused by pathogens present in the drinking water, especially when temperatures rise or the pipes are not hygienically clean, due to inadequate or improper use of biocides.
- But it is obvious and well known that when water contamination is eliminated, intestinal integrity is maintained and good production parameters are obtained, in addition to improving the quality of poultry products.
Bibliography available upon request