Embryo diagnoses must be routinely performed, and not only when problems arise.
Hatchery
Problems and solutions at the Hatchery
To read more content about aviNews May 2020
Hatchery
To read more content about aviNews May 2020
Content available at:
Español (Spanish)
Opening of unhatched eggs remaining on the tray and the assessment of lesions or abnormalities in the rejected fowls during hatching are a significant tool that allows evaluation of the management and conditions fertile eggs were exposed to during a certain period of time in the hatchery.
Mortality occurs during every embryonic stage of development. What is important is to be able to establish what is “normal”, and in case something is not, to be able to determine the cause(s) at that time.
Embryo diagnoses must be routinely performed, and not only when problems arise.
Those very trays where embryo diagnosis is performed must be monitored for water loss upon transfer, hatching window and weight of the chick with respect to the initial weight of the egg.
The chances of being able to spot the cause of the problem are increased when the findings upon opening the eggs are related to the above monitored parameters.
A few cases found in incubation plants, which can be useful to understand future abnormal embryo mortality in our plants are shown next.
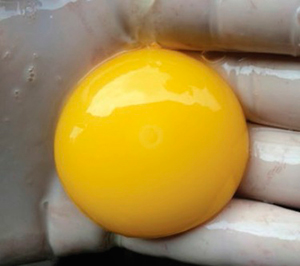
Photograph 1. Fertile, dead before incubation

Photograph 2. Dead on day 1-2 of incubation
Photograph 3. Weak shells
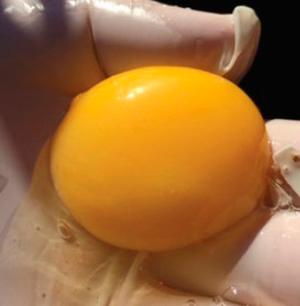
Photograph 4. Mottled yolks

Photograph 5. Contamination
There were no changes in the way eggs were handled in the farm -collection, spraying, storage temperature, or transportation
In this case, early mortality was the result of a severe stress situation (earthquake): mottled yolks (disturbance of the vitelline membrane) were the evidence.
Weak shells, some of them fissured, which lead to contamination, were the result of the stress caused by oviposition before the shell was completed, or an abnormal retention of the egg in the egg formation gland, which resulted in subsequent eggs not having enough time to develop properly.
In this case the existence of an abnormal percentage of red hocks and pipped eggs were observed, as well as the presence of umbilical cords (“navel strings”).

Photograph 6. Red elbows

Photograph 7. umbilical cords
In this case the cause was the excessive moisture in the incubator and hatchery. The high amount of pipped eggs, along with the presence of umbilical cords confirms the diagnosis – Photograph 7
Usually, when the temperature is low the hatching is delayed. A high temperature leads to red elbows, splayed legs and crooked toes, and the hatching window shows an acceleration of embryonic development.
In this case, the existence of a high percentage of chicks with navel issues, such as black buttons, navel strings, and omphalitis.

Photograph 8. Black button navels
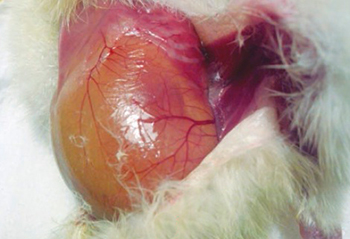
Photograph 9. Omphallitis
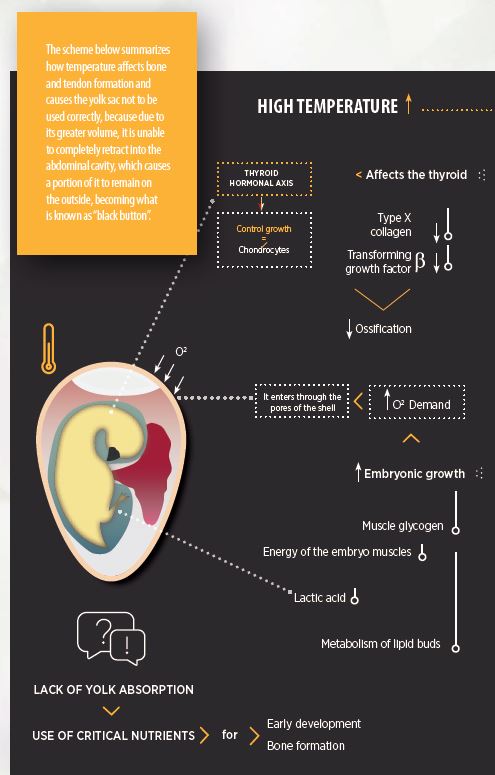
The scheme below summarizes how temperature affects bone and tendon formation and causes the yolk sac not to be used correctly, because due to its greater volume, it is unable to completely retract into the abdominal cavity, which causes a portion of it to remain on the outside, becoming what is known as “black button”.
In this case, a reduced hatchability, pipped eggs, chicks with shells on their feathers, and excess fluff on hatcher doors were observed.

Photograph 10. Pecked egg

Photograph 11. Pecked egg

Photograph 12 Chick with shell attached to feathers

Photograph 13. Down attached to hatchery gate
In this case, several factors had an impact:
In this instance we found a high mortality rate in the farm; the chicks had lost their apetite, they were lethargic due to yolk sac issues and chick motricity.
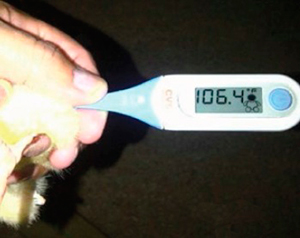
Photograph 14. High temperature during the last week in the incubator and hatchery
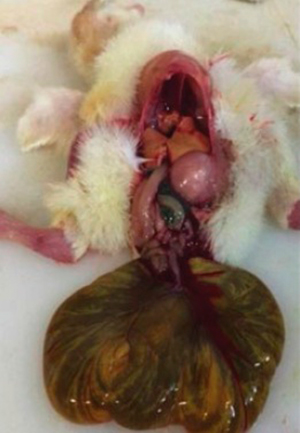
Photograph 15. Fowl with a 20% yolk sac ratio
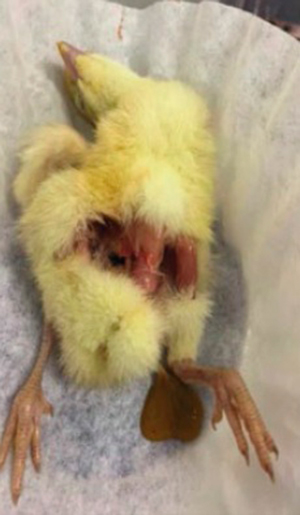
Photograph 16. Fowl with an 8% yolk sac ratio

Photograph 17. Upside down chick
In this case, the temperature during the last week in the incubator and hatcher was too high – Photograph 14 -.
That high temperature affected the absorption of the yolk sac. Chicks with large yolk sacs do not have the need to search for food.
In addition, high temperatures affect the leg bone formation and hence, motricity.
Photograph 15 shows chicks with a yolk sac ratio of 20% in contrast with Photograph 16, which shows fowls with 8% yolk sacs.
The high levels of carbon dioxide and high temperature upon reception can generate “upside down chicks” Photograph 17
The blood samples from affected chicks show high levels of lactic acid and low glucose. Glucose is essential for the brain. Due to the low glucose level the stored glycogen is used, thus generating a great oxygen demand. When there is not enough oxygen available, lactic acid is produced, which is extremely irritant for the chicks’ nervous system.
This situation is more frequent in eggs from the first laying week, since the eggshell is thicker, which makes oxygen diffusion difficult.
The table below can be used as a guideline for assessing cracking in broiler chickens (Tullett 2009).